

DRug Integrative discoVEry for small molecule:
To progress your program from target identification to IND
DRIVE-Small Molecule brings together the right skills, expertise and discovery platforms to design and deliver new clinical chemical entities in therapeutic areas such as oncology, immuno-oncology, and immuno-inflammation.
DRIVE-Small Molecule, a way to drastically speed up your new chemical entities, developed with our strategic partner Zobio.
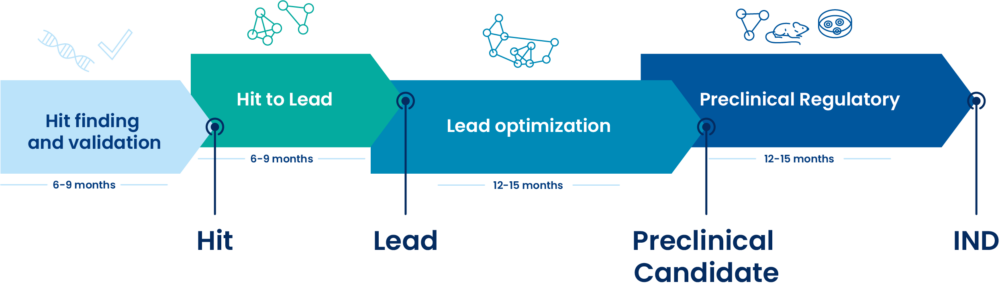
DRIVE-SM offers a complete solution through the entire small molecule drug discovery value chain from hit-finding, hit to lead, lead optimization, preclinical candidate through to IND-filing, aiming to deliver and accelerate the entry of high quality new chemical entities into the clinic.
DRIVE-SM encompasses all drug discovery platforms including multiple hit-finding/screening capabilities, medicinal chemistry, computational chemistry, in vitro biology, ADME / DMPK, in vivo pharmacology, pharmaco-imaging and early safety assessment to deliver drug candidate, particularly in oncology, immuno-oncology and inflammatory/auto-immune diseases.
All the steps for your small molecule’s Research & Development projects
-
Hit finding and validation
The Hit identification and validation phase represents a first critical step in drug discovery. The quality and properties of the hit series will delineate the chance of success at progressing rapidly into developable preclinical drug candidate.
Within the DRIVE-IDDS consortium, we guide and assist you at defining an optimal and customized strategy to identify hits for your preferred target. We provide a range of different and complementary cutting-edge technologies that we can apply to screen for or design novel hits, ranging from DNA-encoded libraries, fragment libraries screening using several biophysical methodologies, and de novo design through AI approaches.
Hit series identified from those technologies and methodologies are judiciously characterized through a defined set of critical assays and filters to enable the selection of most promising hits series with early drug-like attributes.
-
Hit to Lead
The Hit-to-Lead phase is about establishing quickly and rigorously the understanding of the Structure-Activity-Relationships (SAR) of different hit series, through rational design, on multiple essential parameters from the affinity and selectivity against your target, thanks to early ADMET and pharmacokinetics properties.
The major objective at the end of Hit-to-Lead step is to provide a comparative assessment and evaluation of all hit series, in order to select the most promising chemically different series with drug-like properties to undergo subsequent Lead Optimization phase.
In the DRIVE-IDDS consortium, we have been exposed to a number of hit-to-Lead programs on diverse biological targets, many of them being converted into Lead selection decision within a 6-9 months’ timeframe on average.
-
Lead optimization
The key objective of the Lead optimization phase is to identify and select one patentable pre-clinical candidate (and possibly 2-3 back-up compounds) demonstrating a robust profile of activity, selectivity, PK/PD relationships, therapeutic preclinical efficacy – with a good safety margin – and pharmaceutical developability with a scalable route of synthesis to undergo preclinical regulatory IND-enabling studies.
The Oncodesign Services Drug Discovery experts are experienced in implementing and executing multiple parameters optimization plan, with dynamic and adjustable decision trees, and with anticipated de-risking strategies to fine tune the identification of preclinical candidates. Our immediate processing of compound testing undertaken by our experienced multidisciplinary teams in drug discovery, located under one-roof, enables to generate quickly the critical information and results to sustain an efficient and speedy optimization with reduced cycle-times.
Our teams have delivered more than 20 preclinical candidates of high quality over the past years, many of those being progressed in clinical trials.
All intellectual property generated during the course of the hit to lead and lead optimization phases belong to the client
-
Preclinical Regulatory
Within the DRIVE-IDDS consortium, we guide you on the implementation of a well-defined and well-designed preclinical IND-enabling study package to progress your drug candidate into clinical trial in a timely and mastered manner.
Our teams and partners, are used to work under a GLP compliant environment and facilities for the safety and toxicological studies. They will ensure effective planning and execution of the IND roadmap to clinic, adjusted to the targeted clinical indication. This includes, the intended route of administration and integrates the management of the non-GLP and GLP drug substance batches production.
After preclinical candidate selection, we have been able to progress and complete several IND-enabling study packages within an average of 12-15 months.

Hit finding and validation
- DNA-encoded library screening
- Library and HTS screening
- Fragment screening
- AI
- Biophysics SPR, DSF, ITC, NMR
- Biostructural X-ray and Cryo EM
- Assay Development
- Protrein systhesis and purification
Hit to Lead
- Medicinal chemistry
- Structure based drug design / molecular modelling
- AI
- Biophysics SPR, DSF, ITC, NMR
- In vitro biochemistry & biology
- In vitro and in vivo DMPK
- Early safety assessment
Lead Optimization
- Medicinal chemistry
- Structure based drug design/ molecular modelling
- Multi parameter optimization – AI
- In vitro and in vivo DMPK
- In vivo pharmacology & Proof of Concept
- Multi species PK & allometric scalling
- Pharmaco-imaging
- Early scale-up and formulation
- Early safety assessment
Preclinical Regulatory
- Project management and regulatory filing
- CMP synthesis / scale – up
- Pharmaceutical development
- Formulation
- Regulatory toxicity studies
- GLP bioanalysis and PK
Our expertise
A strong alliance of expert
ZoBio has built a class-leading platform that provides a complete solution for Fragment Based Drug Discovery to our clients. Our gene-to-lead capabilities include: protein production, biophysical and functional assays, structural biology (crystallography, NMR & cryoEM) and fragment focused medicinal chemistry. Our team of 46 people brings its dedication and expertise to help solve your largest drug discovery challenges.
Contact an expert to exchange on your project :