Une technologie de chimie médicinale propriétaire
Nanocyclix® est basée sur la macrocyclisation de petites molécules « Lead-like » et sur laquelle OPM appuie ses programmes de découverte de médicaments et de biomarqueurs.
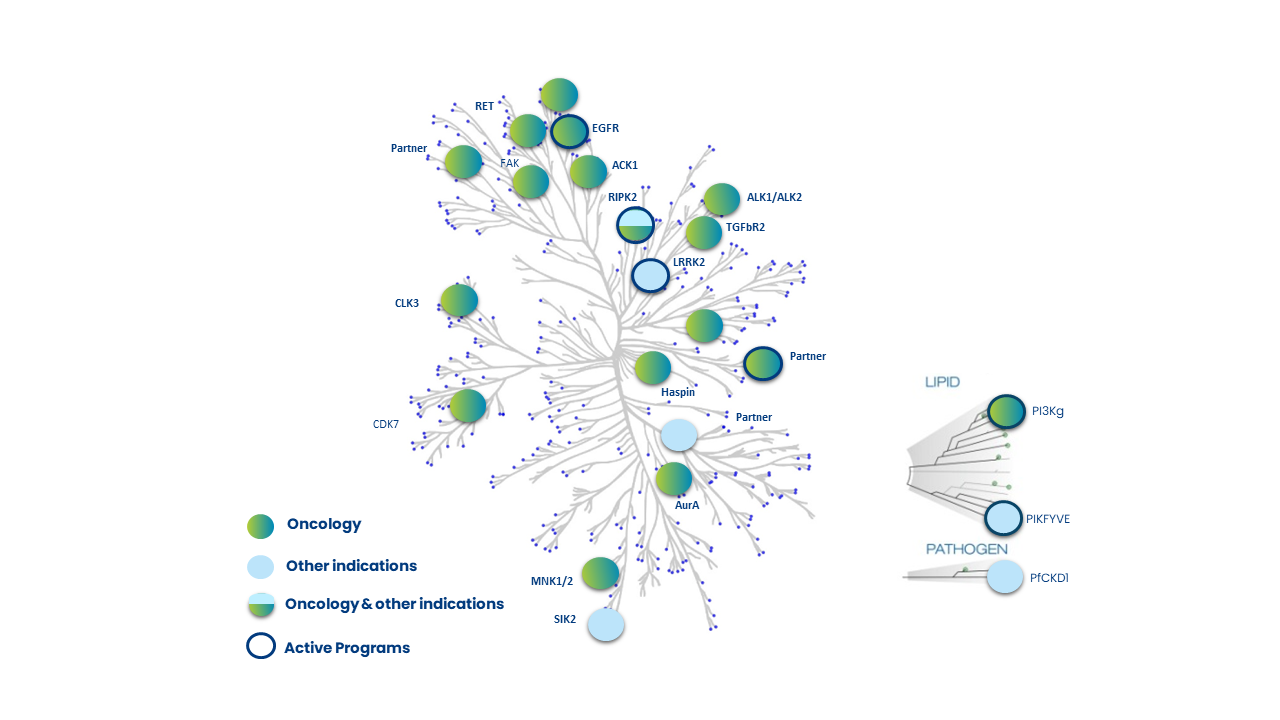
Sur la base de ce principe, OPM a développé une plate-forme complète d’inhibiteurs de kinases macrocycliques sans équivalents dans l’industrie. La plateforme actuelle se compose de plus de 11 000 macrocycles, basée sur plus de 50 « scaffolds » et plus de 300 « linkers » distincts. Ces inhibiteurs de kinases de type I, profilés sur le kinome humain, sont puissants, très sélectifs, et présentent une bonne pénétration cellulaire. Ils présentent par ailleurs des critères de développabilité, physico-chimique et ADMET favorables, avec une SAR très prédictive. Cette approche permet aussi de générer des molécules qui passent la barrière hémato-encéphalique pour des applications dans des maladies du SNC.
Plusieurs leads sont en cours d’optimisation, aussi bien pour des kinases connues que pour des kinases inexplorées ; ils deviendront demain des thérapies ciblées en médecine de précision.
Trois défis à relever pour l’inhibition de kinases
Les kinases jouent un rôle clé dans la régulation de la plupart des fonctions cellulaires, telles que la prolifération, la progression du cycle cellulaire, le métabolisme, la survie / apoptose, la réparation de l’ADN endommagé, la motilité et la réponse au micro-environnement. Le ciblage des kinases – une famille de plus de 500 protéines dans le génome humain qui régulent la signalisation cellulaire par le transfert d’un groupe phosphate de l’ATP à leur protéine de substrat – représente une opportunité majeure dans plus de 400 maladies.

Ils doivent être très puissants pour concurrencer les concentrations élevées d’ATP dans les cellules. Les molécules qui bloquent le site actif dans les kinases doivent avoir des activités de l’ordre du nanomolaire.

La spécificité est essentielle. Les inhibiteurs doivent bloquer la kinase d’intérêt uniquement sans toucher les plus de 500 autres kinases pour éviter les effets secondaires indésirables.

La résolution des deux défis ci-contre tend à conduire à des molécules aux propriétés physico-chimiques qui se prêtent mois au développement de médicaments. Les inhibiteurs doivent se trouver dans ce que l’on appelle un « espace médicamenteux ».
Les molécules Nanocyclix® représentent une opportunité extraordinaire pour le « chemical biology ». Dans cette approche, complémentaire au « target based drug discovery », la diversité des molécules Nanocyclix® synthétisées est testée sur un grand nombre de kinases (« profiling »). Ceci génère des sondes (« probes ») ayant une grande puissance et sélectivité pour des kinases peu explorées ou difficile à cibler (intractable). Cette approche « probe based drug discovery » permet ainsi d’ identifier des opportunités de thérapies « First in Class » sur des kinases peu ou non explorées ou difficiles à cibler. et l’approche de criblage de notre diversité moléculaire sur des kinases d’intérêt ouvre la voie vers des inhibiteurs de nouvelle génération potentiellement « Best in Class ».
Les propriétés physicochimiques (faible poids moléculaire, passage des membranes cellulaires) des molécules Nanocyclix® en fait une collection idéale pour une évaluation dans des tests phénotypiques : des test cellulaires représentant des conditions pathologiques de maladies sans solutions. Les organoïdes générés partant de biopsies de victimes de cancers sans solutions sont un bon exemple. L’identification de molécules Nanocyclix® avec une forte cytotoxicité dans ces modèles, sans effet adverse sur des cellules normales, permet aussi bien d’identifier de nouvelles cibles que de développer de nouveaux traitements.
Le poids moléculaire d’un inhibiteur de kinase Nanocyclix®, même après une optimisation complète, est généralement de l’ordre de 350 à 450 daltons, nettement inférieur à celui d’un inhibiteur linéaire avec une puissance et une sélectivité similaire. Cela génère un profil plus « drug-like » qui est encore optimisé en utilisant le « rational and structure based design » et des techniques d’optimisation multi-paramètres (MPO) de chimie médicinale moderne.
Les molécules Nanocyclix® peuvent cibler avec précision les kinases intraitables et inexplorées dans diverses familles de kinases, y compris les lipides kinases. En raison de leur petite taille et de leur espace conformationnel restreint, les inhibiteurs de Nanocyclix® traversent facilement les membranes cellulaires et traversent même la barrière hémato-encéphalique. Ils sont également sujets au radiomarquage à base de 18F à des fins de diagnostic.
Découvrez aussi





