Proprietary medicinal chemistry technology
Nanocyclix® is based on the macrocyclisation of lead-like small molecules, on which OPM bases its drug and biomarker discovery programmes.
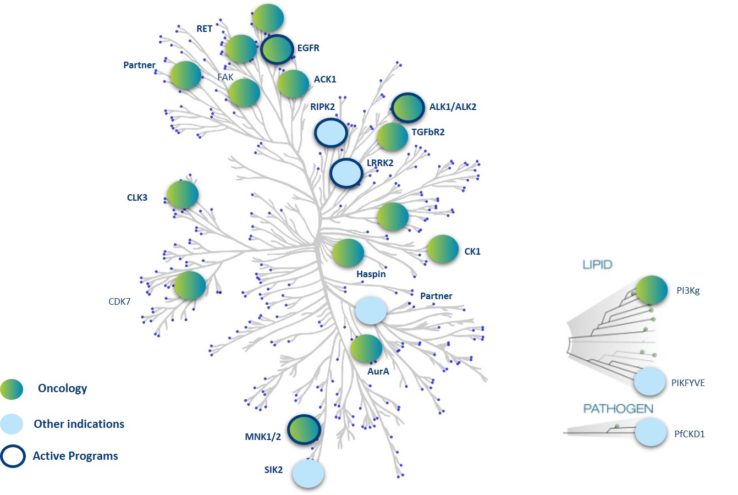
OPM has constructed a full platform of small molecule macrocyclic kinase inhibitors that is unique in the industry. The current platform consists of more than 11,000 macrocycles, based on more than 50 scaffolds and more than 300 separate linkers. These type I kinase inhibitors, profiled using the human kinome, are potent, highly selective, and have good cell penetration. They also boast good developability, physicochemical properties, and ADMET, with a highly predictable SAR. This approach also makes it possible to generate molecules capable of crossing the blood-brain barrier, for applications in CNS diseases.
Several leads are currently being optimised, both for known kinases and for unexplored kinases; they will become tomorrow’s targeted therapies in precision medicine.
Three challenges when it comes to inhibiting kinases
Kinases play a key role in regulating most cellular functions, such as proliferation, the cell cycle, metabolism, survival/apoptosis, DNA repair, motility, and microenvironment response. Targeting kinases – a family of more than 500 proteins in the human genome that regulate cell signalling by transferring an ATP phosphate group to their substrate protein – represents a major opportunity in more than 400 diseases.

They must be very potent in order to compete with high ATP levels in cells. The molecules that block the active site of the kinases must possess activities in the nanomolar range.

Specificity is key. Inhibitors must only block the kinase in question, without affecting the other 500+ kinases, in order to avoid adverse effects.

Compromising between these two challenges tends to lead to molecules with physicochemical properties that are less suitable for drug development. Inhibitors must act within what is known as a “drug space”.
Nanocyclix® molecules represent an extraordinary opportunity for chemical biology. In this approach, complementary to target-based Drug Discovery, the diversity of synthesized Nanocyclix® molecules is tested on a large number of kinases (“profiling”). This generates probes with high potency and selectivity for kinases that are little explored or difficult to target (intractable targets). This probe-based Drug Discovery approach thus makes it possible to identify opportunities for first-in-class therapies on kinases that are little explored, unexplored, or difficult to target, and our molecular diversity screening approach for kinases of interest paves the way to potential best-in-class next-generation inhibitors.
The physicochemical properties (low molecular weight, cell membrane transport) of Nanocyclix® molecules make it an ideal collection for phenotype testing assessment: cell-based assays representing pathological conditions of diseases without an existing solution. Organoids generated using biopsies of cancers with unmet medical needs are a good example. The identification of Nanocyclix® molecules with high cytotoxicity in these models, with no adverse effect on normal cells, allows for both the identification of new targets and the development of new treatments.
The molecular weight of a Nanocyclix® kinase inhibitor, even after complete optimization, is generally around 350 to 450 daltons – significantly lower than a linear inhibitor with similar potency and selectivity. This generates a more drug-like profile that is still optimized using a rational and structure-based design and multi-parameter optimisation (MPO) techniques from modern medicinal chemistry.
Nanocyclix® molecules can precisely target unreachable and unexplored kinases in various kinase families, including lipid kinases. Due to their small size and limited conformation space, Nanocyclix® inhibitors easily cross cell membranes and even the blood-brain barrier. They can also be radiolabelled with 18F for diagnostic purposes.
Also discover




