RIPK2, a kinase of the innate immune system, targeted to treat IBD
IBD is one of the autoimmune and inflammatory pathologies, linked to a deregulation of the immune system.
First indications in IBD for RIPK2:
- Crohn’s disease and ulcerative colitis
Potential to represent a “franchise” target:
- Other inflammatory and autoimmune diseases
- Aggressive and metastatic cancers
RIPK2 is part of a complex activated by bacterial infections, with the purpose of killing infected cells to protect the body.
The pathological hyperactivity of this complex gives rise to inflammatory diseases, especially in the colon.
- 20 to 30% of current reference treatment sales (ENTYVIO sales and its successors) estimated in 2030
- Blockbuster potential(> 1 Md US$)
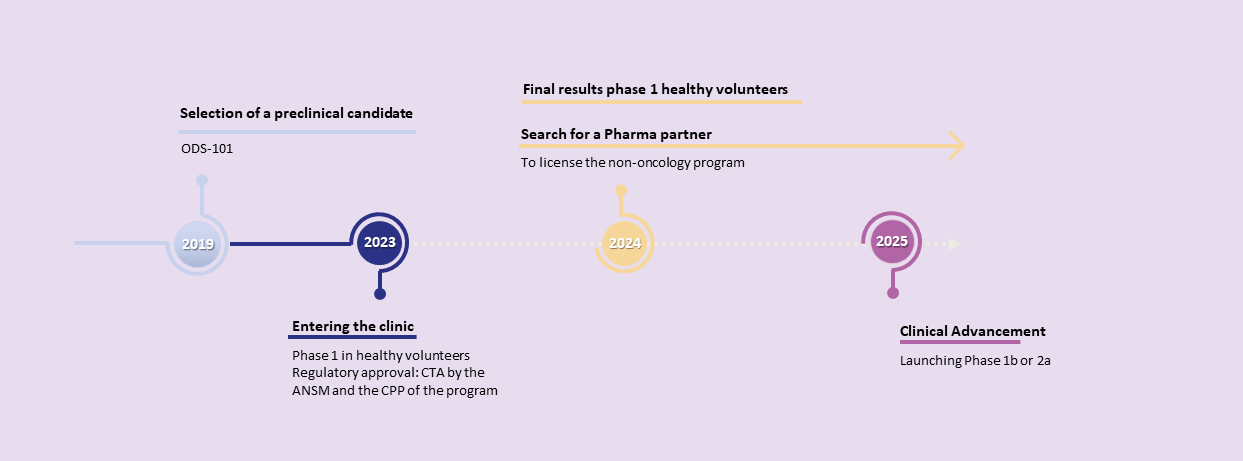
OPM-101 : The leading proprietary program from the Nanocyclix® platform to inhibit RIPK2
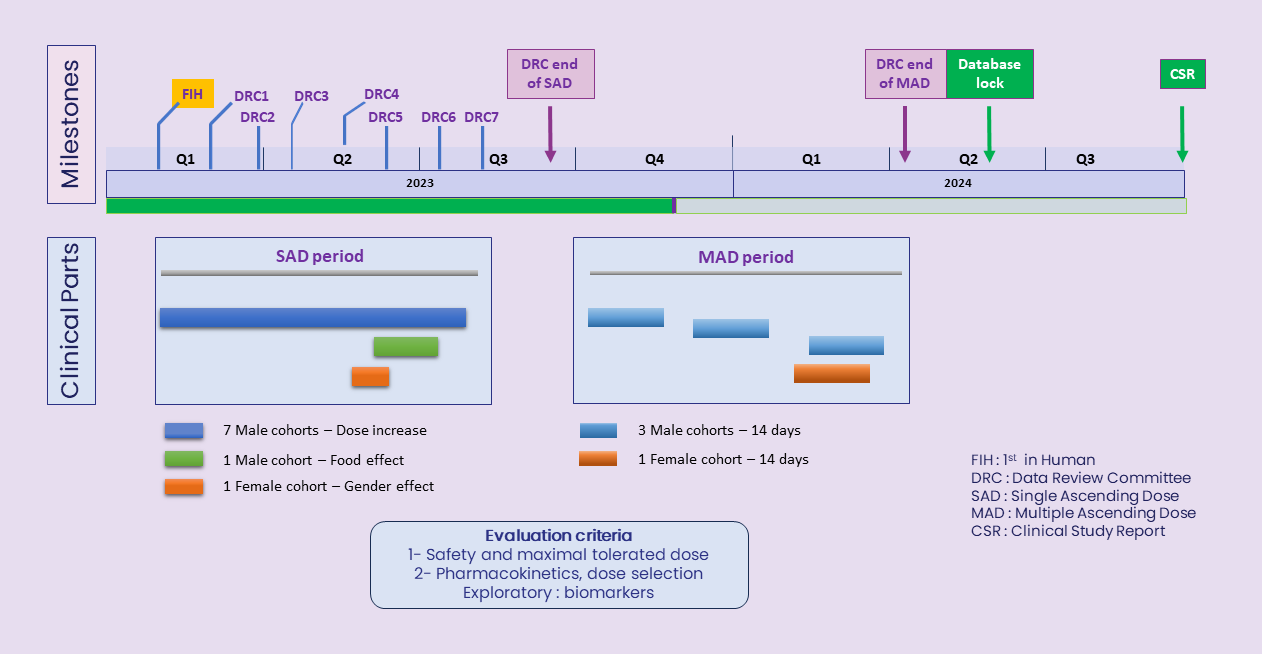
OPM-101 phase I healthy volunteers