LRRK2 inhibitor, a target with the potential to change the course of Parkinson’s disease
Parkinson’s disease is a Progressive neurodegenerative disease affecting ~1% of the population over 60 years old. There are only symptomatic treatments to date and +8.5 million people with Parkinson’s disease worldwide in 2019.
- A mutation in the LRRK2 target was identified in Parkinson’s disease patients in 2004
- A significant increase in LRRK2 kinase activity is observed in vulnerable dopaminergic neurons of Parkinson’s patients, suggesting LRRK2 involvement in most Parkinson’s disease patients
- Biogen/Denali have initiated a Phase 3 trial with their LRRK2 inhibitor BIIB122. Denali’s license to Biogen represented an upfront value of more than US$1 billion.
- Use as a neuroprotective agent and preventive treatment may address nearly 3 million patients in the 7 MM
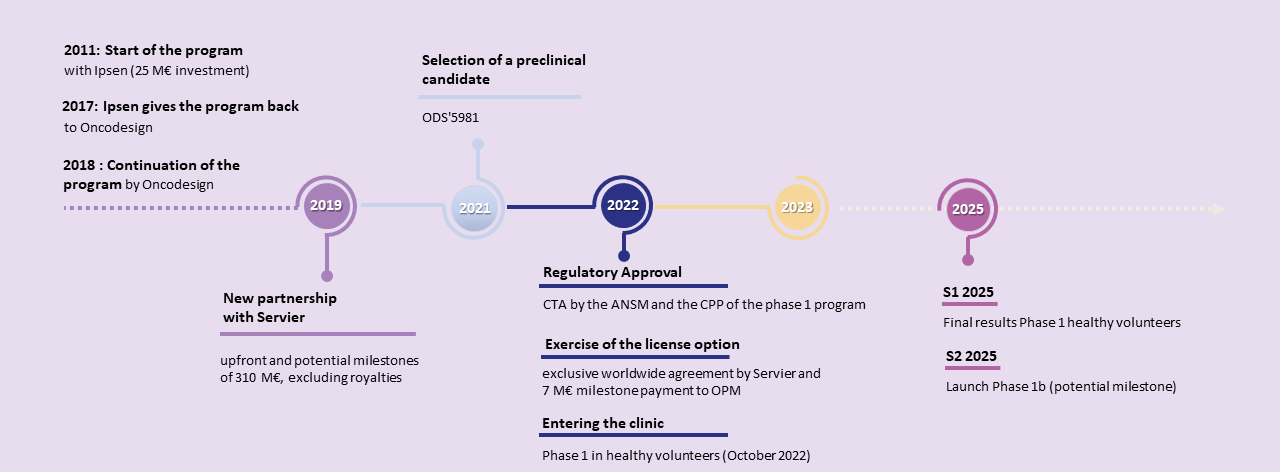
The Phase I healthy volunteer study, initiated in October 2022, has just been completed, confirming the safety of OPM-201 in healthy volunteers.
Final Phase 1 results are expected in the second quarter of 2025.
Read more