The immuno-oncology (IO) market is experiencing robust growth, with the global market for IO drugs—including checkpoint inhibitors—reaching over $109 billion in 2025 and projected to surpass $400 billion by 2034. This surge is driven by rising global cancer incidence, significant investment in R&D, and expanding indications for immunotherapies. Checkpoint inhibitors, especially those targeting PD-1/PD-L1 and CTLA-4, dominate the market and generate the majority of IO drug revenues due to broad approval across solid and hematologic malignancies. However, despite dramatic advances, a substantial proportion of cancer patients still fail to achieve durable responses, primarily because many tumors maintain an immunosuppressive or “cold” microenvironment that blunts the effectiveness of existing therapies.
Current Landscape and Unmet Needs
- Current Therapies:The main checkpoint inhibitors in clinical use include CTLA-4 inhibitors (e.g., ipilimumab), PD-1 inhibitors (e.g., pembrolizumab, nivolumab), and PD-L1 inhibitors (e.g., atezolizumab, durvalumab), approved for diverse cancers such as melanoma, lung, kidney, bladder, and others.
- Patient Needs:
- Persistent unmet needs include extending durable remission rates,
- Overcoming primary and secondary resistance,
- Reducing immune-related adverse effects,
- Offering effective options for patients with “cold” or immune-excluded tumors.
Treatment Gaps: Up to 70% of patients may not sustain long-term benefit from checkpoint inhibitors blockade and require novel, combinatory, or next-generation IO agents
OPM-101 Summary
- Compound class: Small-molecule, highly selective orally available RIPK2 inhibitor
- Mechanism: Selective NOD2/RIPK2 pathway inhibition. OPM-101 is designed to reprogram immune responses in cancer, overcoming key resistance mechanisms and unlocking the full potential of immunotherapy.
- Indication: Cancer patients resistant to immune checkpoint inhibitors
- Clinical Phase: Phase 1 complete (SAD & MAD); Phase 1b/2a in melanoma patients with secondary resistance to checkpoint inhibitors in planning; combination with anti-PD1
- PK profile: Rapid absorption, long half-life
- Safety: Excellent; no serious adverse events
- Target engagement: Robust, sustained TNF-α inhibition even at low doses
- Key differentiator: tackles both resistance mechanisms in tumour micro-environment and neo-antigen presentation on cancer cells
Potential application
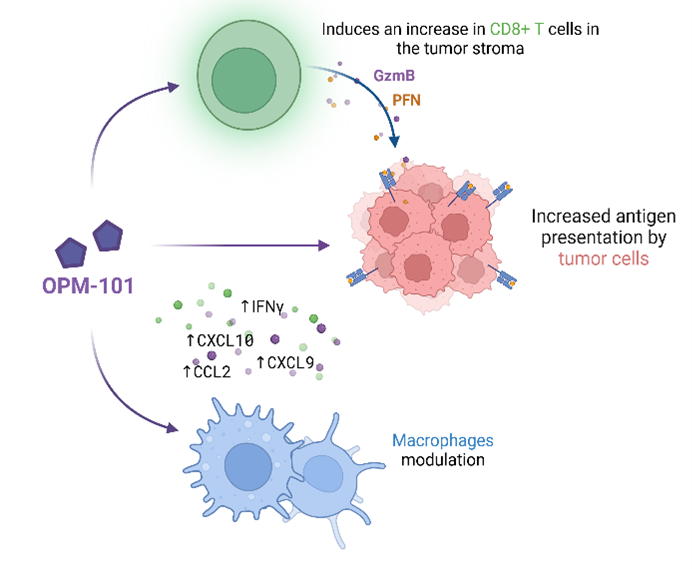
RIPK2 is a central regulator of immune signaling in the tumor microenvironment, influencing innate and adaptive immune cell behavior. Recent research demonstrates that elevated RIPK2 expression in tumors contributes to immunotherapy resistance, particularly by impairing cytotoxic T lymphocyte responses and fostering a suppressive tumor milieu. By inhibiting RIPK2, it is possible to reprogram the tumor microenvironment, restore T cell activity, and sensitize previously unresponsive tumors to checkpoint inhibition. In addition, RIPK2 inhibition has been shown to exert a direct effect on cancer cells by increasing antigen presentation.
In summary, while checkpoint inhibitors have transformed cancer therapy, there remains critical demand for innovative modalities like RIPK2 inhibition to expand and sustain responses in a wider patient population.
Potential effects of OPM-101 in I/O:
1) Modulate tumor microenvironment to higher inflammatory state
2) Increase antigen presentation on cancer cells
Development status: Phase 1: Highlights
- Healthy Volunteers: Single Ascending Dose (SAD) phase with dosing from 5 to 1,000mg (including dedicated food-effect and female cohorts). Multiple Ascending dose (MAD) phase with dosing from 75 to 300mg bid for 14 days.
- Randomized, placebo-controlled, exploring PK/PD, safety, and target engagement metrics.
- Results: OPM-101 shows rapid absorption, long half-life of 12h. Safety is excellent with no serious adverse events, up to the largest doses. Target engagement is robust, with sustained TNF-α inhibition even at low doses
IP status
- Differentiated chemical backbone from other RIPK2 inhibitors based on OPM’s Nanocyclix technology.
- Composition of Matter patent filed in 2016, granted in most countries, including 5MM. Selection invention with OPM-101 and analogs filed in 2021
Licensing Opportunity
OPM-101 is available for out-licensing both globally and for specific regional development, potential asset acquisition worldwide.