LRRK2, a target with the potential to change the course of Parkinson’s disease
Parkinson’s disease is a progressive neurodegenerative disorder affecting movement and non-motor functions, with hallmark symptoms such as tremors, rigidity, and slowed movement.
Globally, the prevalence of Parkinson’s is rising rapidly due to aging populations, with projections estimating over 25 million people will be living with the disease by 2050—a 112% increase from 2021. This growing patient population is driving substantial market expansion; the global Parkinson’s disease treatment market was valued at approximately $5.65 billion in 2024 and is expected to reach between $7.6 and $13.3 billion by 2030–2034, fueled by new therapies and rising demand for advanced treatments. Current therapies are mainly symptomatic in nature, leaving a high unmet need for disease modifying agents. Among these, LRRK2 inhibitors show significant promise, as they target a key pathway implicated in disease progression and may offer the potential to slow or halt the course of Parkinson’s disease.
Industry wide, major efforts have been spent since 2004 in search of safe and brain penetrating LRRK2 inhibitors.
As of today, only 3 molecules have advanced in the clinic, DNL-151 from Denali/Biogen and Neu-411 from Neuron23 are in Phase 2 studies, closely followed by OPM-201 that is Phase 2 ready.
OPM-201 summary
- Fast follower of DNL-151 and NEU-411
- Potential Best-in-Class: Potent inhibitor of LRRK2, Equipotent against WT/Mutants LRRK2, Selective, Brain penetrant
- Small molecule derived from OPM’s proprietary Nanocyclix® technology, oral dosing
- Strong IP position
- Successful FIH Healthy Volunteers SAD and MAD started Sept 2022, finalized Dec 2024 by Servier partner
- Full rights returned to OPM following strategic decision by Servier at the end of 2024
- OPM has regained full control of all data, IP, biology and chemistry samples including >60kg GMP Drug Substance
- Unique “Quick start” partnering opportunity for company/investor with expertise in PD
- Highly derisked program (>120M$ invested), ready to be tested in PD patients
Potential application
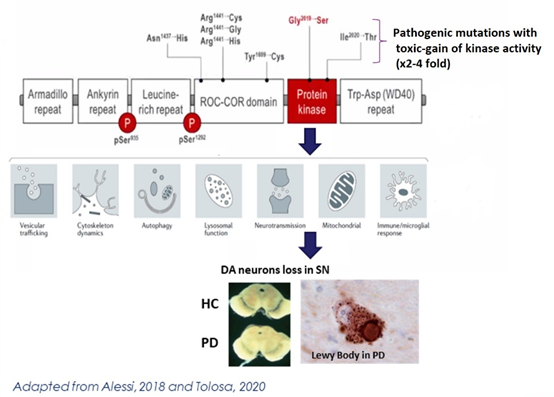
Disease modifying agent for Parkinson’s Disease based on the discovery of an activating mutation of LRRK2 in a familial form of PD.
Importantly, there is increasing evidence that LRRK2 activity may also play a role in a subset of the larger idiopathic group of PD patients without a family history of PD, suggesting that therapies targeting LRRK2 could be beneficial to a broader population than just individuals with rare, familial LRRK2 mutations.
This gives OPM-201 a potential blockbuster potential.
Development status
OPM-201 has finished Phase 1 studies in healthy volunteers, demonstrating good safety and strong target engagement.
>60 kg of GMP Drug Substance is available.
IP status
Composition of Matter patent with expiry date in 2041/42. Patent could be extended with an optimized formulation.
Licensing Opportunity
OPM-201 is available for out-licensing both globally and for specific regional development, potential asset acquisition worldwide.